
Voltaic Pile
October 15, 2016
I found the available information to be sadly lacking in data and graphs and so proceeded to do my part to rectify the situation. The details are in the Instructable I wrote, but here is a brief summary.
For a voltaic battery constructed out of copper and zinc cells the voltage per cell is between 0.9 and 1.0 volts. The internal resistance (with a salt and vinegar electrolyte solution) is about 640 Ohms per cell.
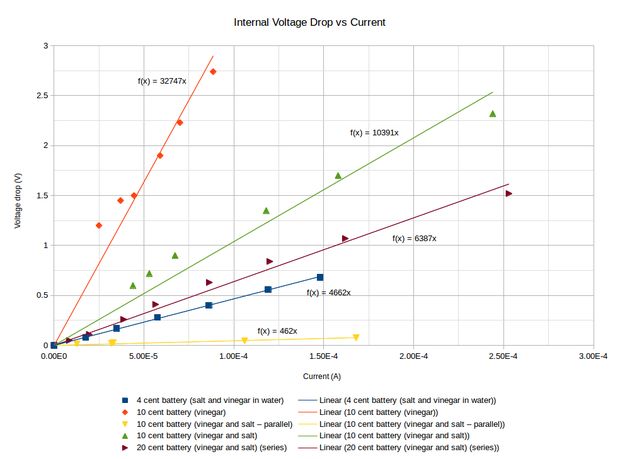
The graph shows how I determined the internal resistance for various situations. Knowing the voltage and the internal resistance, I calculated that it would take 710 batteries each containing 10 cells, where each cell consists of two pennies, to build a battery capable of putting out 5 volts at 0.5 amps, that is, a mediocre phone charger.
That comes out to $142 worth of pennies.